NHSX Digital Technology Assessment Criteria (DTAC) – Compliant and now available via Spark DPS
The Tympa System is compliant to NHSX’s Digital Technology Assessment Criteria (DTAC), with our DTAC documentation available on request for NHS organisations. DTAC covers the following areas:
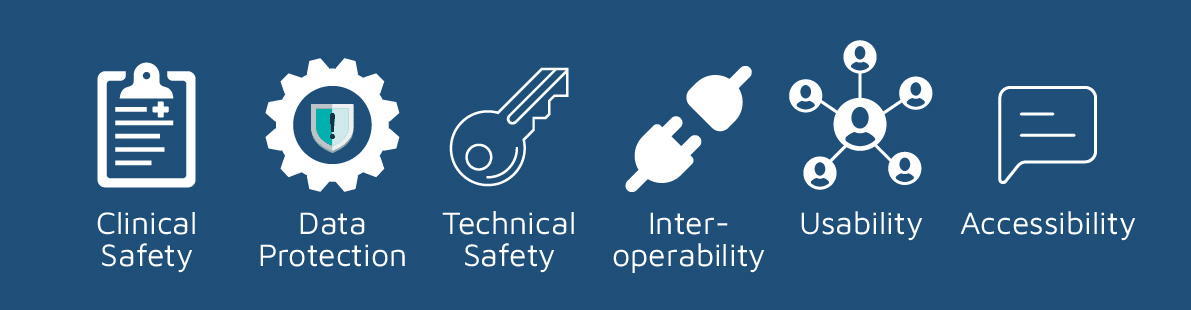
Clinical Safety
We are compliant with the DCB0129 standard for Clinical Safety having assessed and mitigated safety hazards relating to the Tympa System. Our approach to Clinical Safety is underpinned by our Clinical Risk Management plan and articulated in the Clinical Safety Case Report for the Tympa System.
The TympaHealth system is the combination of an intelligent application that incorporates machine learning, software and hardware. As a medical equipment the hardware a CE marked Class 1 medical device and FDA registered. We have a declaration of conformity for this and are registered with the MHRA.
Data Protection
We have met the standards of the NHS Data Security and Protection Toolkit (DSPT), have a Data Protection Officer in place and are registered with the Information Commissioner’s Office. We process all data within the United Kingdom and are GDPR compliant.
Technical Security
TympaHealth are Cyber Essentials certified, run penetration testing at least once annually and have load tested the Tympa System.
Interoperability
For the NHS, the NHS Number is used as the primary identifier for patients, which is subject to appropriate validation. We have put a plan in place for Electronic Health Record integration that will use HL7 and FHIR standards and run securely over TLS v1.2.
Usability
Users of the product and patients are consulted as part of the development process for the Tympa System. This is done through a mixture of one to one communication with customers and focus groups of patients. We are committed to taking continual feedback from our customers and focus groups to ensure our product remains aligned to user needs.
Accessibility
TympaHealth are committed to achieving WCAG 2.1 AA compliance and are currently working towards this. Read our accessibility statement.
Ongoing DTAC compliance
For every new release of the Tympa System we maintain and update our documentation in the above areas, filtering this into our DTAC submission so that it is always up to date.
Tympa System – now available on Crown Commercial Service’s Spark DPS
Tympa Health Technologies Ltd have been named as a supplier on Crown Commercial Service’s Spark DPS framework and are listed in the following categories:
Subject Area – Level 1: Health
Subject Areas – Level 2: Diagnostics, Treatment, Prevention (Health)
Delivery Method – Level 1: Artificial Intelligence
Delivery Methods – Level 2: Machine Learning, Intelligent Applications, Deep Neural Networks, Computer Vision, Ensemble Learning
Delivery Method – Level 1: Data
Delivery Method – Level 2: Human in the Loop Crowdsourcing
Buyer’s can follow this guide to procure the Tympa System from Spark DPS